
Radiation Therapy: External Beams
By Dr. Zoomie
This article is part two of a two-part series on radiation therapy. Read about Brachytherapy: ...
Read morequestions? Call us

By Dr. Zoomie
This article is part two of a two-part series on radiation therapy. Read about Brachytherapy: ...
Read more
By Dr. Zoomie
This article is part one of a two-part series on radiation therapy. Read about external ...
Read more
By Dr. Zoomie
Dr. Zoomie! Going through the cabinets of a few recently retired engineers at work I ...
Read more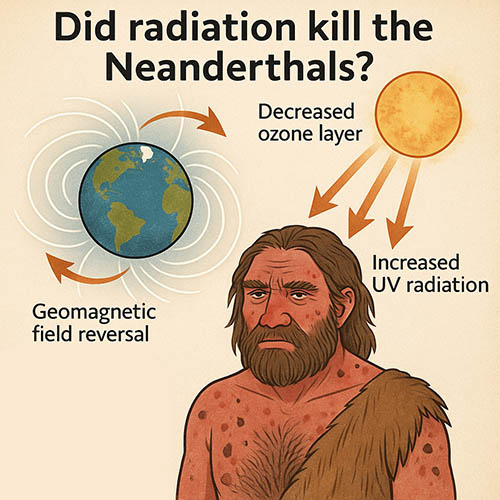
By Dr. Zoomie
Did radiation kill the Neanderthals? So, Dr. Z – I saw something in my feed ...
Read more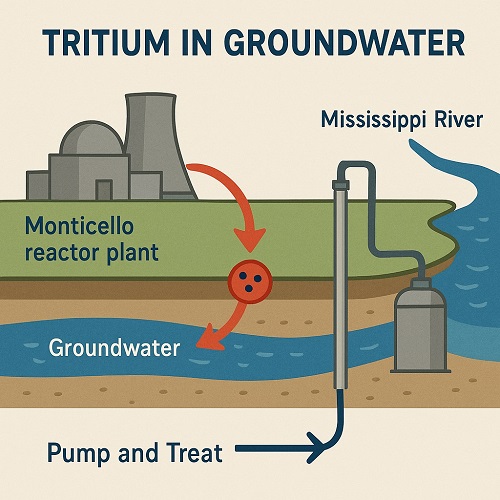
By Dr. Zoomie
Tritium in the groundwater? Hey Dr. Z – I live in Minnesota, near the Monticello ...
Read more
By Dr. Zoomie
Interstellar Fission? I’m puzzled, Dr. Z. I read what you wrote about the natural nuclear ...
Read more
By Dr. Zoomie
Hi, Dr. Z – I was reading through some of our regs the other day ...
Read more
By Dr. Zoomie
Dear Dr. Zoomie – I liked the piece you wrote about how particle accelerators work; ...
Read more
By Dr. Zoomie
Good evening, Dr. Zoomie! I was reading something about being able to study isotopes to ...
Read more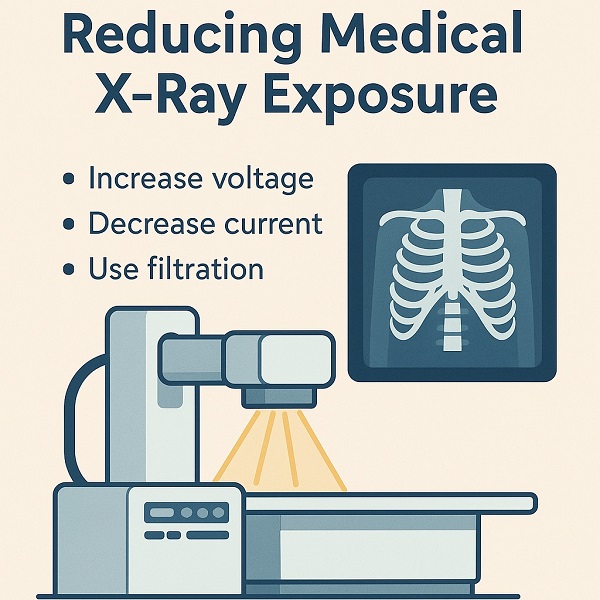
By Dr. Zoomie
Yo, Doc Z! I was wondering if you can tell how they make x-rays and ...
Read more