Does radiation really make things glow in the dark?
Good evening, Dr. Zoomie – I was watching the movie Modern Problems (having an 80s flashback) and there was this one scene where glowing green radioactive waste was leaking out over Chevy Chase – it ended up giving him superpowers. I know the superpowers bit isn’t quite accurate, but it made me wonder – does radiation actually cause things to glow green (or any other color for that matter)?
Well…the best answer for this one is “It depends.”
When I was in the Navy we nukes used to have fun with this one – one joke was that being a nuke saved us money because we didn’t need a night light, and one of the nicknames for Navy nukes was “glow worms.” And we didn’t really do much to dispense with the stereotype because it could be sort of fun. But, sadly, there are limits to what radiation can do – in the words of science fiction author Stuart Hardwick, radiation isn’t “magic death cooties,” neither does radiation exposure make one glow in the dark. Sorry.
That being said, the myth is not wholly unjustified because radiation can cause some very specific compounds to glow while they are being exposed to it – that’s how an entire class of radiation detectors (scintillation detectors) works. So let’s see why radiation makes some things glow, but not others.
Where the whole “green glow” thing started, I think, is with zinc sulfate. I’m not sure who first figured it out, but when alpha particles hit zinc sulfide they cause it to give off photons of visible light – these photons happen to have the right amount of energy to look green (violet photons have the highest amount of energy and red have the least). The radio-luminescent properties of zinc sulfide were discovered over a century ago, and other substances were found to be radio-luminescent over the next few decades. But because zinc sulfite was the first and most popular such compound found, the green glow it produced became identified with exposure to radiation.
Here’s the thing, though – zinc sulfide only glows while it’s being exposed to radiation – as soon as the radiation source goes away, so does the glow. So radiation can, indeed, cause a green glow – but only when there’s both a source of radiation and the phosphor. Take away either of these and the glow goes away as well. Which means that simply irradiating something is not going to make it glow forever – not even for a few minutes – it’ll only glow as long as the radioactive source is present.
Radium paint made this easy – the paint contained both zinc sulfide and radium in a suspension so the alpha radiation given off by the radium could usually find a zinc sulfide molecule to excite to give off the glow. But, again, if we could separate these two components, the glow would stop.

There are other ways that radiation can cause a glow. Scintillation-type radiation detectors (such as zinc sulfide) will emit photons of visible light when exposed to radiation, and the color of light emitted changes depending on the compound and the type of radiation to which it’s exposed.
Several years ago, for example, I was holding a material (I can’t remember exactly what it was) that was glowing a pretty bluish-purple when under bombardment with alpha particles. And I’ve seen the bluish glow of water due to a phenomenon called Cherenkov radiation when looking into the core of an operating nuclear reactor used for research purposes as well as when I was looking into a pool that contained newly added spent reactor fuel.
In the case of the scintillation materials the explanation isn’t very difficult – when radiation strikes the scintillator (zinc sulfate for alpha, the plastic anthracene for betas, or sodium iodide in the case of gammas) the radiation adds energy to the molecules it runs into, causing electrons to jump up to a higher energy level. The electrons drop back down to where they prefer to be very quickly and when they do so, they give off a quick flash of light to account for the energy they lost – sort of like the way that a ball you drop will give off energy in the form of sound when it strikes the ground.
Cherenkov radiation is more interesting – it’s given off when a charged particle (an electron, for example) is traveling faster than the speed of light in air or water or whatever medium the radiation is passing through. There are actually telescopes that are designed to detect the Cherenkov radiation emitted when ultra-high-energy cosmic rays slam into the Earth’s atmosphere – they detect the faint glow of photons emitted when these particles, travelling just shy of the speed of light in vacuum, enter our atmosphere where the speed of light (in air) is a little lower. It’s also emitted when very high-energy beta particles pass through glass (in which light travels more slowly than in a vacuum). I have to admit that I don’t fully understand the physics behind this, but I can vouch for the effect, having seen it with my own eyes a few times.
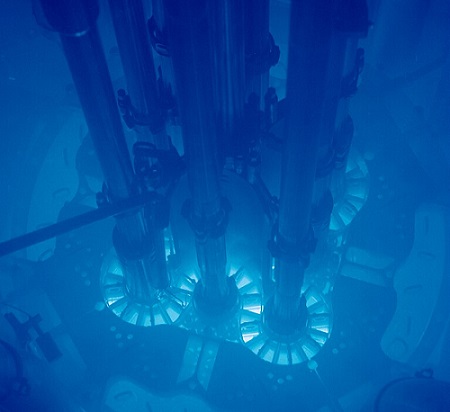
Author: Argonne National Laboratory, Source: https://commons.wikimedia.org/wiki/File:Advanced_Test_Reactor.jpg
Here’s the thing – if something is glowing then it’s giving off energy and the only way that can happen is if there’s energy being pumped into whatever it is that’s glowing. If radiation is being absorbed by a scintillator then the radiation is providing that energy. If radiation is going through water or air, causing Cherenkov emissions then, again, the radiation is providing the energy. On the other hand, if I am exposed to radiation while, say, I’m working with radioactive sources, but then I put the sources away and go home…now there’s nothing adding energy to me so I’m not going to glow because the radiation was in the past and it’s no longer adding energy to my body. Or, to put it another way, things that are being exposed to radiation might glow, but things that have been exposed to radiation will not.
The other thing is that to get a good glow we need more than a source of energy – we also need something that will glow when it absorbs that energy. That’s why radioactive waste (like what they showed in the movie you mentioned) doesn’t glow. The radioactivity gives off energy, but the stuff the radioactive waste is made of doesn’t give off light. I’ve produced and handled – literally – tons of radioactive waste over the years, but never in a form that glowed. Sorry – I know it’s not very exciting, but that’s the way it goes. That being said – the few times I’ve seen the purplish glow from a scintillator or the blue glow of Cherenkov radiation and the many times I’ve seen the green glow from radium, tritium, or promethium luminescent paint – it always looks very cool!
