Dear Dr. Zoomie – I just heard that we’re always exposed to radiation and it sort of scares me. And on top of that, there’s the radiation from Fukushima. Can I just line my walls with lead? And how much lead do I need to cut down this radiation to something safe?
Well…I think you might be overly concerned here. First, you’ve got to remember that radiation from natural sources has been a part of life on Earth since it first formed – it’s safe to say that every single organism that has ever lived on Earth has been exposed to radiation. Not only that, but there’s ample evidence that these natural background radiation levels today are the lowest they have ever been. This means that today we are being exposed to less radiation (from natural sources) than were our distant ancestors.
It’s also important to realize that natural radiation varies considerably from place to place on Earth. Some places – the American Gulf Coast, for example, most of Hawaii, Japan, and so forth – have unusually low levels of natural radiation (more on this in a moment) while other places (Ramsar Iran, the American Colorado Plateau, Guapari Brazil, and others) have elevated levels of natural radiation. Interestingly, cancer rates don’t seem to be at all correlated with natural radiation levels. For example, the parts of the US with the highest natural radiation levels also have among the lowest cancer rates in the country. Some people have actually used this fact to suggest that low levels of radiation might make us healthier – I’m not sure I can agree with that, though, because there are so many other things that can affect cancer rates. For example, the population along the Gulf Coast is generally older than in the Rocky Mountains, there are more people who smoke, and the area has a significant petrochemical industry – all of these could serve to increase cancer rates compared to the Rocky Mountain states. The most we can say is that the effects of these different radiation levels is fairly low – low enough to be swamped by the other factors.

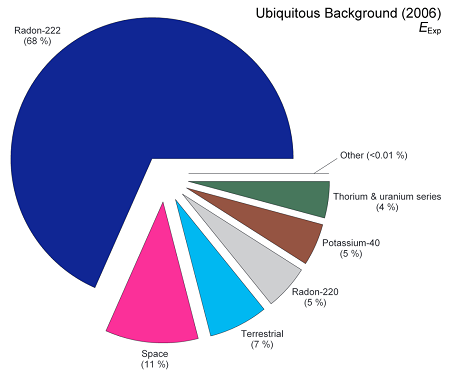
As far as where this natural radiation comes from, there are four main sources, and these all change from place to place. They are:
- Radiation from the rocks and soils. All rocks and soil contain potassium (about 0.01% of potassium is naturally radioactive) along with trace amounts of uranium and thorium. Uranium and thorium, in turn, decay through a number of radioactive progeny until they reach stable lead – these progeny include radium, radon, and polonium. So any rock or soil that you analyze is going to have radioactivity in it and we’re all exposed to radiation from this. On average, we receive 20-30 mrem annually from radioactivity in the rocks and soils as well as from building materials made from them (for example, bricks are made of clay that an contain radioactive potassium).
- Radon in our homes and buildings. Radon comes from the decay of uranium in the rocks and soils. Radon is a gas; once it forms it will seep through the soil and into our homes – since it’s heavier than air it collects in basements, subway tunnels, caves, and so forth. Places with higher levels of uranium and thorium in the rocks and soils will have higher concentrations of radon in the air. On the other hand, since radon is such a heavy gas it’s not a concern above the first floor of any building. We receive about 150-250 mrem annually from radon, depending on the local geology and the amount of time we spend in areas where the radon might collect.
- Radiation from our own bodies. As mentioned earlier, a tiny fraction of potassium is naturally radioactive, including the potassium in our own bodies. In addition, we’re always exposed to radioactive carbon (carbon-14) and hydrogen (H-3, also called tritium) that’s formed in the atmosphere from interactions with cosmic rays. Finally, we’re always ingesting or inhaling trace amounts of dust that can contain uranium or thorium. All told, we receive about 40 mrem each year from all of these sources of radiation in our own bodies.
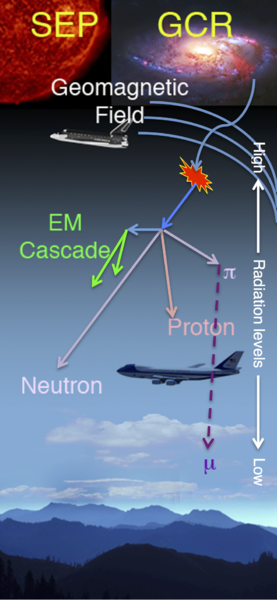
- Cosmic radiation from the sun and stars. The sun gives off high-energy particles as the solar wind. In addition, distant stars give off their own stellar winds and some of those particles escape into space as well – some of these stars, by the way, are far more active and energetic than our sun and the particles they give off have far more energy than what our own sun emits. As if that weren’t enough, every few decades a massive star explodes in our galaxy in one of the most energetic events in the universe – a supernova. The particles from these explosions also permeate space. When any of these particles – whether from our sun or from elsewhere in the galaxy – slam into our atmosphere they can generate what are called cosmic ray air showers; cascades of particles and gamma rays that percolate down to the ground to expose us to radiation. In general we receive from 20-40 mrem each year from cosmic radiation.
Putting all of this together, we receive about 250-300 mrem each year from natural radiation, with some variability depending on where we live and what we eat. And remember – first, there’s no way to get away from this and second, we’ve been exposed to this radiation for as long as there has been life on Earth.
So – as far as your questions go – lining your walls with lead might help to shield you from cosmic rays, but that’s only a minor source of radiation. You might also line your floors with lead to reduce dose from the rocks and soils, but that’s also somewhat minor. Sealing your basement might remove radon as a source – but there’s no way to eliminate the radiation exposure from the radionuclides in your own body. But really, this doesn’t matter – again, this is radiation that our bodies have the ability to deal with. The bottom line is that I really don’t see a reason to try to shield your home from natural radiation. Ah (I hear you say) but what about the radiation from Fukushima? For that, read on!
There’s no denying that the Fukushima meltdowns put a lot of radioactivity into the environment. A lot of it ended up in the Pacific Ocean and a lot settled out on the ground in Japan, but a lot of radioactivity ended up in the atmosphere. This was measurable in the US – just as fallout from the Chernobyl nuclear accident could be measured in the US. In fact, after the Chernobyl accident I was on a nuclear submarine that was stationed off the coast of the Soviet Union and we could measure the radioactivity in the air whenever we came up to ventilate. But what I learned then – and what is important to realize now – is that just because we can measure the radioactivity doesn’t mean that it’s a threat. With the right equipment, for example, I can measure the radioactivity in my own body, in the granite countertop in my kitchen, or in a bunch of bananas. That’s because we’re really good at measuring radioactivity. But, again, just because we can measure it doesn’t mean that it’s dangerous. Consider – I can measure the weight of a single grain of sand or a single particle of dust. But does that make it dangerous to have a grain of sand land on my head? Not really – because the weight of the sand grain is so small that, although measurable, it’s insignificant. Similarly, while we could measure radioactivity in the air after the Fukushima accident, the radiation dose to anyone in the US was trivial – even in Alaska and Hawaii. Some of the Fukushima radioactivity undoubtedly settled on the ground here, but the majority of that was relatively short-lived iodine (I-131 to be precise) and the remainder was present in such low quantities that it simply poses no more threat than the natural potassium that’s already in the soil.
Finally, I wanted to mention something else briefly – about environmental sampling. Every now and again you’ll read a report that somebody has, say, sampled rainwater or soil, or even made radioactivity measurements on a beach and they’ve found elevated readings. To be honest with you, I always take these with a grain of salt because making these measurements isn’t as easy as one might think. It’s not uncommon to take a quick reading and to get counts that are higher than background readings. But just because you get a reading above background doesn’t necessarily mean anything. To get into a full discussion would require talking about counting statistics, and I won’t subject you to that! For now, let it suffice to say that radiation is random – if you take a radiation detector and push the button to record every count that comes in during the next minute you’re going to get a number. Push it again and you’ll get a different number; push it a third time and you’ll get something else. Over time you’ll notice that the readings are all clustered together a shape that’s called a bell curve. Sixty-eight percent of the time the readings will fall within one standard deviation of the average value and 95% of the time the readings will be within two standard deviations. As long as particular reading is within two standard deviations of the average then you probably don’t have anything unusual. But there are a lot of people who think that any reading that’s above background is significant, when it could simply be that your detector was hit by a cosmic ray air shower while you were counting.
Another mistake that people make is taking too small a sample and counting it for too short a period of time. If you have elevated readings after counting, say, a 1 liter sample of rainwater for an hour, it’s a lot more compelling than having an elevated reading following a 1-minute count of a one-milliliter sample. This is another problem that people run into – they’ll take a small sample, count it for a minute, and if the count rate is a little higher than background then they start worrying about contamination. Most of the time, though, counting a larger sample for a longer period of time (many environmental samples are counted for over 12 hours) will show us that there’s really no contamination. OK – so let me put all of this together!
First, every creature that has ever lived on Earth has been exposed to background radiation and we’ve evolved to cope with this level of exposure. Background radiation comes from radionuclides in the rocks and soils, in our own bodies, from radon, and from cosmic rays. Cosmic radiation varies around the world, but these variations don’t seem to be correlated with health or cancer risks. That being the case, you really don’t need to worry about shielding your home to reduce exposure from background radiation. And with regards to radiation from Fukushima, some of that was detected in the air shortly after the accident and some might even have settled to the ground in the US. But radiation dose from this fallout was so low that it’s not worth worrying about – less exposure than if you were to move from the Gulf Coast to the Rocky Mountains. Finally, there are a number of people who have reported measuring higher levels of radioactivity at various times. But unless the samples were obtained and analyzed by someone with experience in environmental sampling they’re likely to be small samples counted for a short period of time – as such, the results are likely to be meaningless. And even if an increase was seen, unless it’s more than a few standard deviations higher than background levels, the results are most likely nothing more than a statistical anomaly rather than an indication of contamination.