Interstellar Fission?
I’m puzzled, Dr. Z. I read what you wrote about the natural nuclear reactor in Oklo, Africa and it made sense that something like that would work. But I just read that astronomers think that there’s nuclear fission taking place in outer space and I don’t know how that would work since there’s no water to slow down the neutrons and it seems like it would be hard to get uranium at all, let alone in the right configuration. Can you help me understand what’s happening?
Wow – this is really cool! I had to do some searching online to find this, but it’s in one of the best scientific journals in the world (Science), which makes me inclined to believe the results are real. After reading through the paper I think I’ve got an idea of what’s going on – let’s see if I can explain it. And it’s really cool!
So…I think I’ve written before about nucleosynthesis – how hydrogen fuses to create helium, which fuses to form carbon, then silicon, and so forth up to iron. There’s also what’s called supernova nucleosynthesis; when a star explodes there is so much energy that heavier atoms slam into each other to form super-heavy atoms (e.g. lead, uranium, plutonium, and more). On top of that, a supernova is a very neutron-rich environment and these atoms are being bombarded with neutrons as well, forming even heavier isotopes through what astronomers call the “r-process” (“r” stands for “rapid” as this all happens in a handful of seconds).
The combination of superheavy elements plus lots of neutrons is a recipe for fission and that is what astronomers say they’re seeing. They’re not seeing the fission itself (although fission does give off gamma radiation); what they’ve seen are fission products – radioactive fragments of the superheavy atoms that split apart.
You might recall that, in the aftermath of the Fukushima accident, a lot of isotopes of iodine and cesium were released – we saw something similar after Chernobyl and Three Mile Island. We keep seeing these nuclides because of the physics of fission, which results in U-235 atoms splitting in a predictable manner – most of the fission products have masses of around 100 and around 130 neutrons and protons.
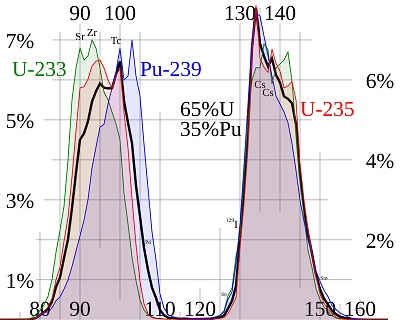
Pu-239.
Knowing that U-235 or Pu-239 was fissioned, we know what fission products to look for; alternately, we can look at the pattern of fission products and understand which atoms were split. This is what the astronomers did.
When a star explodes the debris is scattered across the skies, traveling outwards over hundreds or thousands of parsecs. The debris can initiate shock waves in stable clouds of gas and dust, triggering a collapse that can lead to the formation of stars and planets, and the debris, including the heavy r-process elements and fission products will become incorporated into theses stars and any residual dust.
What the astronomers did was to analyze the amount of 30 different r-process elements present in 42 ancient stars in our galaxy; what they found was a pattern of elements that most likely came from the fission of heavy and super-heavy atoms, including atoms as heavy as 260 atomic mass units, heavier than any naturally occurring atoms on Earth. Such large atoms will not only fission when struck by neutrons, but will also fall apart on their own, undergoing spontaneous fission, similar to the californium-252 (Cf-252) neutron sources I’ve used at times in the past. The difference is that the Cf-252 in the sources I’ve used was produced in high-energy accelerators while the super-heavy atoms that gave rise to the fission products that were observed in the stars that were studied.
So fission is taking place among the stars, but it’s different than the fission that takes place in nuclear reactors. In a reactor the fission goes on for an extended period of time, sustaining itself by producing neutrons keep the chain reaction going – what’s called a self-sustaining chain reaction. This is possible because the fission takes place in a carefully constructed geometry, engineered to make a self-sustaining reaction possible. In space, however, the heavy and super-heavy atoms are produced in the same blast that produced the neutrons causing them to fission while the expansion of the debris and the brevity of the blast shuts down the fission reaction almost as soon as it begins. It’s like the difference between logs burning in the fireplace and the explosion of a hand grenade.
So it does seem as though there’s nuclear fission taking place naturally out there in the universe, but only fleetingly.
