NUREG 1556 Vol 9, Rev 3 (2019), NRC regulation: 10 CFR 20, 10 CFR 30, 10 CFR 35
RSO qualifications, training, and facilities (10CFR35.50)
Education: BS, MS or PhD, science or tech field RSO Training: 40-hour RSO
Experience: 5 years or more in health physics, and pass certification exam in an applicable field; possibly up to 200 hours of formal training
Others: ARSO, Authorized Users, Medical Physicists, and Nuclear Pharmacists must be approved by regulators and certified by appropriate boards
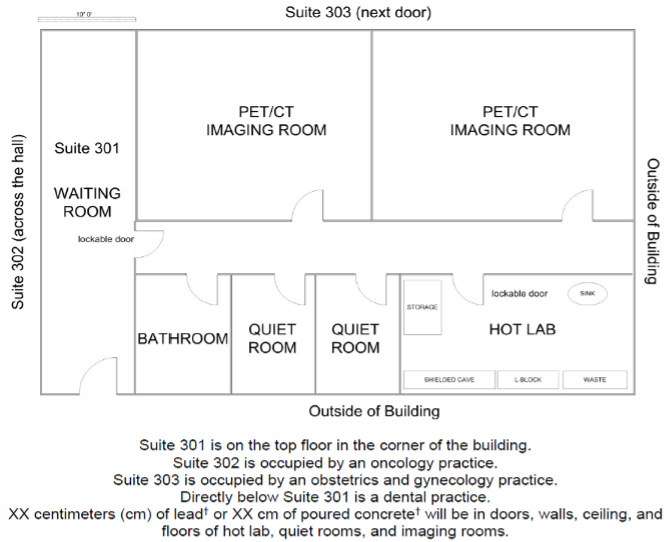
Facilities: Must be adequate to protect health, minimize danger, and secure sources against loss, theft, or damage.
Radiation Safety Program Components
Dosimetry: External dosimetry for all rad workers, bioassay for I-131 users
Instrumentation: Ion chamber or energy-compensated GM for dose rate; pancake GM, gamma scintillation detector for contamination. Calibrate annually.
Surveys: (10 CFR 35.70) Daily dose rate surveys, contamination surveys as noted in license application and license
Source inventory: Maintain inventory of radiopharmaceuticals; inventory and leak test dose calibrator check sources as noted in license (generally every 3 or 6 months).
Program audit: Annual (by internal or external auditor)
—
Procedures: Required to develop and use security procedures, operating procedures and emergency response procedures for each type of fixed gauge
Radioactive package receiving: Required to have SOP for receiving radioactive materials.
Other considerations: Survey hands and feet for contamination before exiting Hot Lab.
Records retention
The information below shows retention requirements for some of the required records. For a comprehensive list, see NUREG 1556 volume 9, Appendix X.
Receipt records: 3 years following transfer or disposal of source
Source transfer: 3 years following transfer or disposal of source
Inventory: 5 years
Disposal: Until license terminated
Important to decommissioning: Until site released for unrestricted use
Dosimetry: Until license terminated
Example Nuclear Medicine suite
Download this profile: