Dear Dr. Zoomie – I saw something the other day that scientists found a natural nuclear reactor that’s over a billion years old. Is this for real? Or was this left behind by aliens?
Never fear – there was an actual natural nuclear reactor found on Earth and aliens had nothing to do with it! It was in a place called Oklo, in what is now the nation of Gabon in western Africa. And what happened is pretty cool.
There’s not enough room here for all the details; if you’re interested in some of the nitty-gritty you can check out an on-line article written by a scientist who is conversant with both geology and nuclear reactors. But here’s the big picture – let’s start with the geochemistry of uranium.
Uranium dissolves easily into oxygen-rich water but not at all into water that lacks oxygen. Until about two billion years ago the Earth’s atmosphere was oxygen-deprived which meant that surface water couldn’t dissolve uranium. Ancient algae was producing oxygen, but all the oxygen that was produced was immediately sucked up by iron and other metals in the Earth’s crust – in effect, the Earth was rusting. About two billion years ago the iron was all oxidized and oxygen began to accumulate in the atmosphere – as soon as it did so it also started to accumulate in rainwater (and streams, lakes, and rivers) and when that rain fell on granite rocks (granite tends to have elevated levels of uranium compared to other rocks) it began to dissolve the uranium out of the water.
As the uranium-rich water flowed along it sometimes came to areas where, due to decaying organic matter (more of that algae) the water was oxygen-deprived; when that happened the uranium came out of solution. And in one place in particular, apparently a fluke, enough uranium collected in one place in a configuration that resembled that of a nuclear reactor – a number of lumps of uranium dispersed in a sandstone formation. And when that area became flooded with water (water slows down neutrons, which makes them more efficient at causing fissions) these lumps of uranium began to fission. As they did so they produced heat, neutrons, and fission products (when a uranium atom fissions it produces two radioactive atoms). Of course, there’s more to making a reactor than mobilizing uranium and precipitating it out of solution in lumps. The uranium also has to be enriched so that it will sustain a nuclear chain reaction – that can’t happen today because there simply isn’t enough of the fissionable U-235 in uranium to sustain a chain reaction. At least, not today. But in the past things were different.
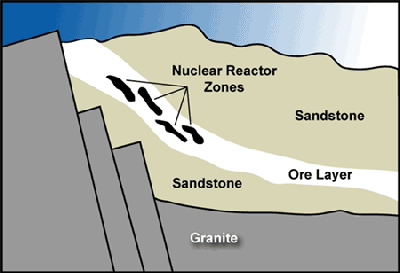
Uranium today has a very specific composition – if you take a uranium sample from anywhere on Earth and count the atoms you’ll find that 99.2% of the uranium atoms have a weight of 238 atomic mass units (it’s abbreviated U-238), and U-238 doesn’t fission very well. Virtually all of the rest (0.72%) of the uranium atoms are a little lighter with a mass of 235 – U-235 fissions quite nicely, but uranium that only has seven tenths of one percent of U-235 will not sustain the chain reaction necessary to keep a reactor operating. This is why we need to enrich uranium; so that there’s enough U-235 to achieve and maintain criticality. And, incidentally, in a nuclear reactor “critical” simply means that the reactor is operating at a constant power – all reactors are critical when they are operating. Anyhow – natural uranium today can’t sustain a chain reaction unless we use something like heavy water or graphite to help coax things along. But two billion years ago, things were different.
U-238 has an incredibly long half-life – it takes almost 4.5 billion years for half of it to decay away, which means that over the entire life of our planet only half of the U-238 it formed with is left. On the other hand, U-235 has a half-life of “only” about 700 million years – there was around 75 times as much U-235 on Earth when it first formed compared to today. And two billion years ago – at the time that the Oklo reactor formed – U-235 comprised about 5% or so of natural uranium; this is about the same amount that’s found in the fuel for commercial nuclear reactors today.
Oklo could not have happened at many times in history. Earlier in Earth’s history there was enough U-235 but not enough oxygen to mobilize the uranium; later in Earth’s history there was plenty of oxygen but not enough U-235 to sustain a chain reaction. But for one brief moment – actually for about a half-billion years – conditions were about perfect for uranium to dissolve, move, and concentrate in a manner that would sustain a criticality. During that time, a body of water-saturated sandstone with a number of uranium deposits achieved criticality.
The reactor operated only sporadically – it depended on water to sustain the chain reaction and, as the reaction progressed, the water would boil away, shutting down the reactor. So the reactor would operate and then shut down; while shut down it would cool off until water could re-saturate the sandstone and the reaction would start up again. Each time this happened a little more U-235 would fission and the concentration dropped slightly – eventually there was too little left to sustain a chain reaction and the reactor shut down for good. All in all scientists estimate that the Oklo reactor operated for about 100,000 years.
Now let’s fast-forward a few billion years to the mid-1970s. French geologists located a rich body of uranium ore and they started evaluating it as a source of reactor fuel. But when they started feeding the uranium into their uranium enrichment system they found they weren’t getting the amount of enrichment they expected – some good scientific detective work showed them that the ore itself was deficient in U-235, something that had never been seen before. Eventually they accepted that this uranium ore body was different than any other on Earth. Then they saw evidence of fission products in the ore. It seemed reasonable to assume that the presence of fission products was linked to the lack of U-235 – when they looked at the geology they had to accept the conclusion that they had found a fossil nuclear reactor – nature (not aliens!) had preempted Enrico Fermi (the Italian Nobel laureate who built the first artificial nuclear reactor) by a couple of billion years.