Dear Dr. Zoomie – I was at a meeting of our local HPS chapter last week and overheard someone talking about Ramsar Iran, saying it’s got sky-high radiation levels. Is this from Iran’s uranium enrichment program? Or maybe related to nuclear weapons or something? Do I need to be worried? Let me know – and thanks!
Wow – I haven’t thought about Ramsar for a long time and your question brings back all sorts of memories. I visited Ramsar a little more than 20 years ago and it was fascinating. You’re correct that, as far as natural radiation levels are concerned, Ramsar has got very high levels – the world’s highest for a populated area (there are some beaches with higher rad levels, but nobody lives there). And it’s all natural – no uranium enrichment required. Here’s what’s going on.

A mile or so beneath the shores of the Caspian Sea is a geologically active area – this is an area that, a few hundred million years ago, was shoved skyward by plate tectonics to form the Elburz Mountains. During this mountain-raising, magma chambers were formed and filled deep underground – some of this magma erupted through the region’s volcanoes and some of it simply cooled and crystallized over time. As magma solidifies minerals crystallize in distinct patterns – the first minerals form at high temperatures and they tend to include the smallest atoms and to form minerals that are rich in iron, magnesium, and other metals, removing these small atoms from the remaining melt. The last minerals to form contain the largest atoms – and, as it turns out, these largest atoms include uranium, thorium, and potassium (about 0.01% of potassium atoms are radioactive), with the remaining melt (and the minerals that form from it) become increasingly radioactive as crystallization proceeds.
But it’s not just the uranium, thorium, and potassium – over the ages the uranium and thorium decay to form additional radionuclides, including radium, radon, and more than a dozen others. These accumulate over millions of years, making the residual magma and the rocks it forms ever-more radioactive – this is what’s been happening in the rocks beneath Ramsar. But how does the radioactivity make its way to the Earth’s surface?
Ramsar is famous in Iran for its hot springs; water from the surface percolates down a kilometer and more, it’s warmed as it passes through rocks that retain residual heat from the ancient magma chamber, by the geothermal gradient, and by the energy of radioactive decay. The hot water rises again to the surface, bringing with it ions of calcium, radium, carbon, and more –dissolved from the rock through which the water passed. What it does not carry with it is uranium because, in descending so deeply the water has lost its oxygen and, while the solubility of many elements depends on the pH of the water, the solubility of uranium depends on the amount of oxygen the water carries; the water that reaches the radioactive rocks can dissolve the radium and other decay products of uranium but it’s unable to dissolve the uranium itself. As the water rises it cools; as it cools the dissolved calcium, carbon, and oxygen precipitate out of solution, crystallizing as calcium carbonate – freshwater limestone, also called travertine.

This is where the geochemical properties of radium come into play – radium is chemically very similar to calcium and when the travertine precipitates out of solution the occasional calcium atom is replaced by one of radium. This happens often enough that some of the travertine in Ramsarhas as much as one million times as much radium as do normal rocks and the dose rates can be higher than 10 mR/hr (I measured one rock that was reading more than 14 mR/hr when I was there).
Of course, the rock weathers to form soil so the soil is also rich in radium. On the other hand, this weathering takes time and radium-226 has a half-life of only (on a geologic time scale) 1600 years. So the soil has less radium than does the rock, the amount of radioactivity is lessened by time and diluted by the organic material, the moisture, and the other minerals of which it is comprised – soil that I analyzed had about 1000 times as much radioactivity as you’d expect to see in such an area. Although the local plants have not been analyzed (at least, not that I know of) it’s likely that some of them take the radium up through their roots and into the tissues, to be eaten by those with gardens; well water, too, contains some radium although the concentration in both food and drink varies considerably from place to place.
The reason for the variability is that hot springs are evanescent phenomena – they form, they flow, and eventually they are clogged by accumulating mineralization, and then the water shifts to a new location. A map of Ramsar shows a patchy distribution of radiation levels – low levels for brand-new hot springs that rise with increasing age and degree of mineralization, then decaying away when the hot spring shuts down.
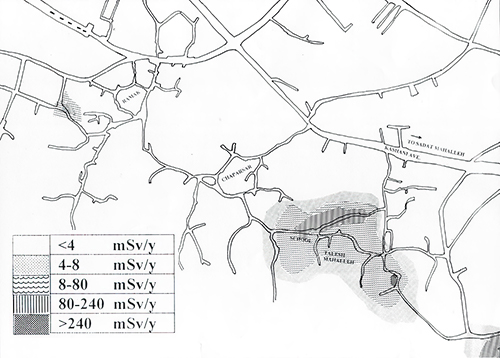
As the radium decays it forms radon, which seeps through the soil and into the air and into homes. Scientists from Iran, Europe, and the US measured radon concentrations as high as 25 pCi/l outdoors (significantly higher than the EPA’s standard of 4 pCi/l in basements) with indoors concentrations even higher due to the use of local rock in building many of the homes in this area. Thus, everyone walking the soil, eating the plants, drinking the water, and breathing the air in these parts of Ramsar are exposed to radium and radon – both of which emits alpha radioactivity that is highly efficient at wreaking havoc in our cells. Estimates are that people living in Ramsar are exposed to as much as 25 rem each year – 1000 times as much as the average person receives from geologic materials and over 100 as much as the typical person receives from radon inhalation.
This, of course, makes one wonder how all of this affects the health of the people living in Ramsar. The answer, according to the scientific literature, is “not at all.” Studies performed over the last few decades show no apparent increase in birth defects, no increase in rates of cancer, and no reduction in lifespan compared to other Iranians living in the area.
There are a number of places in the world with high levels of natural radiation; Ramsar is the highest populated place, but there are others in Brazil, China, India, Australia, and more. And we see the same apparent lack of health impact in the other areas as well. Which could make one wonder if, maybe, these levels of radiation simply aren’t dangerous…but that’s a more complex question than we can address here; that’s a subject for another posting.
