Dear Dr. Zoomie – we’ve got to do some environmental sampling and the lab asked if we wanted to have alpha or gamma spectroscopy done. It sounds important, but I’m not sure what they mean or what they’re used for. Can you help me out?
Spectroscopy can be incredibly valuable, but only if you need to use it. We’ll get into that in a minute – first, let’s talk about what it is. So let’s start with the science part and then go to when you might need to use it.
First – radionuclides give off alpha, beta, or gamma radiation. For reasons that are too complicated to get into here, beta radiation isn’t amenable to spectroscopy – but alpha and gamma radiation are.
Second – we can analyze the alpha and gamma radiation to see how much energy the radiation has. Every nuclide gives off radiation with very specific energy. Cesium-137, for example, gives off a gamma ray that has exactly 662 thousand electron volts (662 keV) and cobalt-60 gives off two gamma rays with energies of 1170 and 1330 keV. These are as specific as fingerprints – anytime you see a gamma with an energy of 662 keV you can bet that it comes from Cs-137. One way to think about it is that every alpha- and gamma-emitting nuclide has a distinctive “fingerprint” of radiation where the identifying features are the energies of the emitted radiation. So if we can measure the radiation energies – alpha or gamma – then we can identify exactly what radionuclides are there.
Third – it’s relatively easy (and not terribly expensive – no more than a few tens of thousands of dollars) to perform gamma spectroscopy, but the cheap and easy way of doing it isn’t tremendously precise. To do gamma spectroscopy well costs over $100 K and is best done by a radiochemistry laboratory. Alpha spectroscopy is another kettle of fish entirely – there is (as of now) no quick, easy, or cheap way to do alpha spec – you pretty much have to have a trained chemist and a full-blown laboratory with a few hundred thousand dollars’ worth of equipment.
The graphics here are from some gamma spectra I made in early 2016 – the top one is Cs-137 and the bottom one is Co-60. Looking at these, it’s easy to see how the gamma spectrum can be used for radionuclide ID – you can do the same thing with alpha radiation; I just don’t have the equipment (or the training) to make these measurements in my lab.

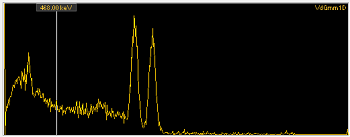
OK – so this is how spectroscopy works; the next question is when you might need to use it. Here are some examples.
Several years ago a rail car filled with scrap metal set off a radiation detector at a steel mill. The gondola car was sent back to its point of origin, which happened to be just a few miles from where I was working at the time. The scrap metal dealer didn’t have the ability to figure out what was causing the alarm – it might have been natural radioactivity or it might have been something manmade; nobody knew how to handle the material until the radioactivity could be identified. Using gamma spectroscopy we were able to identify the source of radioactivity as radium (specifically Ra-226), which made it possible to find a disposal site.
Another example was with one of my consulting clients – they measured elevated levels of radioactivity at a site where they had used thorium in decades past. We had to find out if the elevated radiation levels were due to their operations with thorium or something else. By identifying the specific isotopes present (using both alpha and gamma spectroscopy) we were able to show that my client hadn’t contaminated the site with thorium; the extra radioactivity was naturally occurring and came from some coal-processing that had taken place on the adjacent site nearly a century earlier. This meant that it wasn’t due to any actions of my client and, not only that, wasn’t anything that required remedial action.
There are tons of other examples I could give but they all come down to pretty much the same thing – there are times that you need to know exactly what types of radioactivity you might (or might not) have and the best way to make this ID is through alpha or gamma spectroscopy. If you have to do a lot of it then you might want to do the work yourself, otherwise it makes more sense to send samples off to a commercial lab. One caution – gamma spectroscopy is fairly inexpensive, but alpha spec can cost a few hundred dollars per analysis and a thousand dollars or so to completely analyze a sample. But if the alternative could call for spending hundreds of thousands – or millions – of dollars in cleanup and/or waste disposal, this is money well-spent.