I noticed recently a flurry of articles about Japan’s recent announcement that it planned to release millions of gallons of water from the site of the Fukushima reactor into the Pacific Ocean. Most of the “flurry” was about the response to this announcement – from environmental groups, some of Japan’s neighbors, and other concerned parties. And it made me wonder why so many were so exercised about so little a risk. But before getting into that, let’s take a look at this tritium stuff.
The first time I came across tritium was when I was in the Navy – we had a tritium monitor in the Torpedo Room of my submarine and I asked why. “It’s for the special weapons” was the reply. Which makes sense – tritium is used in some forms of nuclear weapon and if any of it leaked out then we needed to know. I read up on it and realized it wasn’t much of a threat, so I stopped giving it much thought.
Fast-forward to after I got out of the Navy and was working for an academic radiation safety program to help pay for my college. I noticed that a lot of our research labs used tritium so I learned a little more about it. I found out it emitted a very low-energy beta particle – so low-energy that we couldn’t even detect it with our normal Geiger-Mueller detectors; so low-energy that the tritium beta would barely (if ever) even penetrate through the outer layers of skin to reach the living cells underneath. In fact, I remember holding a small plastic vial that contained (according to the label) over 20 curies of tritium – a large amount of radioactivity – and I couldn’t get a single peep on my Geiger counter. And even if someone ingested or inhaled some of it, this same low-energy beta particle made it about the most innocuous type of radioactivity any of us could take into our bodies. It just wasn’t a big deal.
A few years later I was working for the state government and I learned a little more about tritium – mostly that the same low-energy beta that made it such a minor threat also made it hard to clean up since nobody could survey for it directly. Not only that, but tritium, as a nuclide of hydrogen, would simply dissolve into water and would go wherever water or water vapor traveled. My boss, for example, had needed to deal with a concrete wall that was contaminated with tritium; concrete is somewhat porous and the tritium had permeated the entire thickness of the wall and the only way to “decontaminate” the wall was to tear it down entirely. Not because the tritium posed a risk to anybody – the beta particles couldn’t even escape from the concrete to expose anybody (and, one can hope, nobody was nibbling on the concrete itself) – but because the amount of tritium contamination exceeded regulatory contamination limits. So the wall was ripped down, loaded into waste containers, and shipped off to be buried in a waste disposal site. A lot of money was spent to mitigate a non-risk.
Let’s jump ahead a little more – to another job I had that included oversight of the radiation safety at a laser fusion research facility. They had more tritium than I’d ever seen at a single location – enough to kill anyone who ingested or inhaled any of it – and even there we didn’t get any readings from their stores. On the other hand, if they took a tiny (1 mm) plastic sphere, filled it with frozen tritium, and slammed it with a powerful laser a tiny fraction of the tritium would fuse, releasing enough energy and enough radiation to give a fatal dose to anyone unlucky enough to be in the target chamber at the time. Enough of anything can be dangerous – we just have to know where the boundary between “most likely safe” and “possibly unsafe” lies.
Somewhere along the line I also came to understand where tritium comes from – in nature it’s formed when cosmic rays collide with atoms in the atmosphere. The Earth actually has a lot of natural tritium; every glass of water we drink, every lake or ocean or pool we wade into, every tub or hot spring in which we soak has tritium dissolved in it, to the tune of a few tens of picoCuries (pCi) per liter of natural water. Globally, cosmic rays produce over one and a quarter million curies of tritium every year; couple that production rate with the rate at which it decays and we find that our planet has a total inventory of tritium of about 26 million curies, including the tritium that’s in our own bodies from eating, drinking, and breathing.
Tritium is also, of course, produced in nuclear reactors – the core of a reactor is a neutron-rich environment and as water passes through it’s exposed to those neutrons. When they strike a hydrogen atom, sometimes a neutron will stick, forming deuterium (a stable isotope of hydrogen with one neutron and a proton). If another neutron hits a deuterium atom and is captured then it will form tritium (one proton and two neutrons) – this is where the tritium at the Fukushima site originated.
And that brings us to the water to be discharged from Fukushima into the ocean. It turns out that there are about 20,540 curies of tritium contained in the 820,000 cubic meters of water stored at the Fukushima site. Most of this is groundwater from the area, contaminated with reactor coolant that leaked from the broken reactors. But the Pacific Ocean is huge – it contains about 400 billion times as much water as is being stored on the Fukushima site. So adding the water from Fukushima into the Pacific Ocean increases the tritium in the ocean by only a minuscule amount – by less than a trillionth of a curie (or about 1 pCi) for every cubic meter of water in the ocean. And because tritium emits so low-energy a beta particle, this increases radiation exposure by so little (less than 1 microrem annually) that it poses no risk to anybody – or to any creature – in that water. It’s like adding a few more grains of sugar to a pitcher of KoolAid. This means that seafood lovers can continue eating their sushi or their fish-and-chips without worrying that it’s going to hurt them.
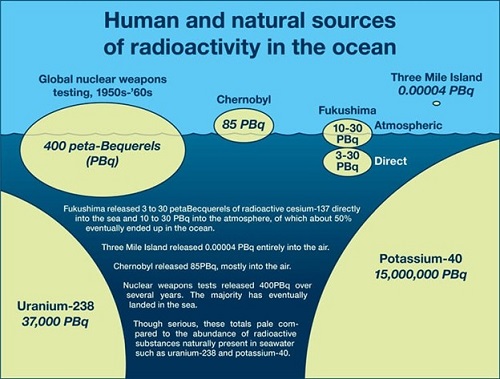
Something else to keep in mind is that seawater also contains many other natural radionuclides that also swamp any radiation dose from the waters of Fukushima. Uranium, rubidium, and potassium are also dissolved in this water, and each of these contributes far more radioactivity (and produces far more radiation dose) than the tritium from Fukushima. The bottom line is that, while discharging this water might be something that we can measure and calculate, it’s just not worth worrying about – in fact, the stress from worrying is going to be more dangerous than the cause of that worry.
Reference: https://www.whoi.edu/multimedia/source-of-radioactivity-in-the-ocean/