So there’s this rock that’s sitting about a meter from where I’m sitting right now. It’s got a beautiful deep green color and it’s a mass of flat squarish crystals that are maybe 5 mm on a side and about 1 mm thick. It’s also radioactive, which is why I bought it at the Columbus (Ohio) rock and mineral show a few decades ago – it’s a uranium mineral called torbernite; this particular piece came from Morocco.

Figure 1: my torbernite-encrusted rock
Knowing that it’s radioactive I was eager to take some measurements when I got it home – and, as a radiation safety professional, I’ve got my own instruments. I can’t remember the readings when I first bought the rock, so give me a minute to grab my instruments and I can get some readings now. Ready for some numbers?
At a distance of 1 cm my Geiger probe gives me a count rate of about 250,000 counts per minutes – a respectable count rate. But I’ve got additional detectors – let me see what they say. My “baby” sodium iodide detector (the crystal on this one is 1 inch tall and an inch in diameter) gives me a reading of 140,000 cpm – less than the Geiger counter.

Figure 2: My GM (in the clip on top of the meter) and my two sodium iodide detectors
I’ve got a larger sodium iodide as well – 2”x2”, or about 8 times the volume of the smaller crystal. That one gives me nearly a half-million counts per minute. The background count rates on each of these (that is, the count rate when the probes are away from any radioactive materials) are about 75 cpm for the Geiger counter, about 3500 cpm with the baby sodium iodide, and about 10,000 cpm with the larger crystal. Or, to summarize them in a table:
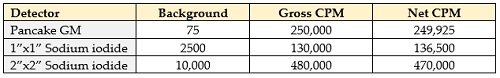
Interestingly, the meter that I’ve connected these detectors to has a faceplate that reads out in mR/hr as well as in CPM. I’ll explain the difference in a minute; for now, let it suffice to say that the dose rate is more important than the count rate if I’m trying to figure out how dangerous this rock might be.

Figure 3: the faceplate of my radiation detector. CPM is on the top and the other two scales are for dose rate.
And – bonus! – I’ve also got a different meter that measures dose rate as well. So let’s see what readings I get from all of these:
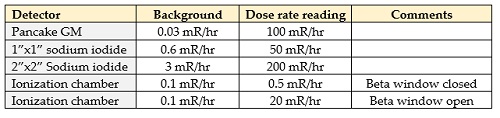
Both of these tables show an awful lot of variability – it can make a lot of people wonder how we can ever know exactly what numbers to use and what these numbers mean.
Let’s look at the count rates – the first table – first.
In particular, take a look at the background count rates – a paltry 75 cpm for the Geiger counter and a whopping 10,000 cpm for the large sodium iodide detector, with the smaller sodium iodide in between. The reason for the difference here is that a Geiger tube is very sensitive to beta radiation and it’s not very sensitive at all to gamma rays; sodium iodide, on the other hand, does a good job of measuring gammas but not so much with beta particles. So looking at these readings tells us that background radiation mostly consists of gamma rays – which makes sense because beta particles can’t travel more than 20 feet through the air at best, and mostly not even 5 feet. So our two gamma detectors are picking up the background gamma rays, which mostly pass through the Geiger tube without registering. And, of course, the 2”x2” sodium iodide detector has a higher count rate because it has four times as much cross-sectional area as the smaller one.
Now let’s look at the meter readings – and this one surprised me, to be honest. The Geiger tube had a higher count rate than did the sodium iodide…but why? One thing that comes to mind is that the Geiger counter is sensitive to beta radiation and the sodium iodide isn’t. This suggests that the torbernite is giving off both beta and gamma radiation – the Geiger tube will measure almost all of the betas and some of the gammas, while the sodium iodide is only seeing the gammas. So my rock must be giving off both beta and gamma radiation. In fact, there’s probably some alpha radiation being emitted as well, but I don’t have a working alpha detector at the moment (mine’s out for repair) so I can’t check to see how much. As far as the larger sodium iodide detector – we see the same factor of four difference between the baby detector and the larger one, so this is just due to the size of the detectors again.
When we look at the dose rate, though, the numbers are all over the place – none of these numbers are dangerous, but since radiation dose affects our risk of getting cancer or radiation sickness it’d be sort of nice to know which (if any) of these numbers we can trust. And part of the key here is to look at the ratios of the readings between the Geiger counter and the two sodium iodides. See anything familiar here? Once again you see that the dose rates are proportional to the readings – the GM dose rate is twice that of the small sodium iodide and the large sodium iodide dose rate is four times that of the smaller detector. In other words, the meter is just looking at the count rate and converting that to dose rate. It’s easy to do – the problem is that it’s the wrong way to measure dose rate.
Here’s why.
Say I throw a piece of gravel at you – you’re upset so you throw a rock back at me. “Not fair!” I say. “Your rock’s a lot bigger than the gravel I threw at you.”
You reply “You threw one thing at me and I threw one thing back at you – we’re even.”
“But yours hurt me more than mine hurt you.”
And that’s the thing – the meter that I have these different probes connected to was calibrated with Cs-137, with a gamma energy of 662,000 electron volts (or 662 keV). In effect, my meter has been “told” that every time it sees a count, the radiation causing that count has an energy of 662 keV. But the radiation coming from my rock has a slew of energies – some have more energy than Cs-137 and most have less. And that’s why the readings are so varied – my meter has no idea how much energy is passing through it – it only sees the number of counts. And since radiation dose (and dose rate) are related to the amount of energy deposited in a material, this particular meter can only accurately measure radiation dose rate from Cs-137 and it’s going to be wrong for everything else. Or, to put it another way, I don’t trust any of the first three readings.
But the ion chamber – that’s different. The ion chamber can tell the difference between high and low energies, so the readings take into account the higher and lower gamma energies from my rock. And – even better – there’s a piece of plastic on the bottom of the meter!

Figure 4: my ion chamber; the case (left) shows the brown beta shield that can be slid down to measure beta dose; the beta radiation passes through the foil window on the bottom of the black ionization chamber on the right.
I know – exciting, right? But here’s the thing – when that piece of plastic is covering a thin metal window on the bottom of the meter it screens out the beta radiation that we know is coming from the rock; when the window is open then the betas can enter the chamber as well. So the dose rate with the beta window is open is 40 times as high as when the beta window is closed – that confirms what we concluded with the count rate measurements – there’s more beta radiation being given off than there is gamma. Cool, right?
It’s also good to know what these readings mean – what’s normal, what should be investigated, and what might hurt us. Let’s start with the easy one – count rate.
If I’m measuring count rate it’s usually because I’m looking for contamination – contamination is only very rarely a health risk, it just affects whether or not we need to wear protective clothing or decontaminate ourselves, equipment, or areas. So as long as the count rate I’m measuring isn’t high enough to call for decontamination then I don’t worry about it all that much. And unless the count rate is really high – in the hundreds of thousands of counts per minute, the contamination doesn’t pose much (if any) risk.
Most of the time, in a non-emergency setting, we want to try to keep contamination levels to a minimum. So anytime I have more than about a few hundred cpm above background with a GM or more than about 1000 cpm above background with a 1”x1” sodium iodide I’ll stop to clean it up (I don’t do contamination surveys with the larger detector because it’s too hard to see low levels above background). But in an emergency – a nuclear reactor accident or a dirty bomb, for example – we can actually let people have as much as 100,000 cpm with a Geiger counter before we need to start cleaning things up. And after the Fukushima accident, there were so many people who were contaminated that the Japanese changed their limits from about 10,000 cpm to over 100,000 cpm without causing any added risk to the public.
With dose rate, I normally measure less than 0.1 mR/hr with my ion chamber – and more like 0.01 mR/hr with a suitably sensitive instrument. When dose rates get to about 2 mR/hr the public isn’t allowed to have unrestricted access – but nobody’s going to be harmed by this level of radiation. In fact, it’s not until the dose rates get into the tens of thousands of mR/hr that they start to pose a risk. Here’s a subjective summary:
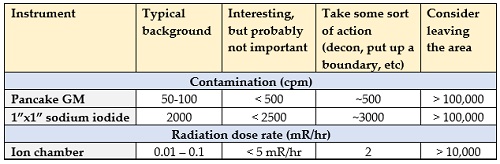
The last thing to mention here is the units of radioactivity, what they mean, and when they start to become a concern.
The biggest thing to understand is that when we’re talking about radioactivity we’re talking about the rate at which the atoms in a source are decaying away. By definition, one curie (the American unit) of radioactivity will undergo 37 billion decays every second (the SI unit, the becquerel, undergoes one decay every second). This has nothing to do with the size of the source, by the way. One gram of radium (Ra-226) has one curie of activity – the same amount of radioactivity as three tons of the much longer-lived depleted uranium, and the same activity (still one Ci) as 100 micrograms of tritium or one milligram of cobalt (Co-60). This means that you can’t just look at a radioactive source and judge the risk it poses by its size – one gram of Co-60 can give you a fatal dose of radiation in less than an hour; a ton of depleted uranium is radiologically safe. The only way to judge the risk a gamma source poses is by making radiation measurements with your trusty ion chamber.
Of course, you might be able to find a label that gives the activity of a radioactive source, there might be a sign on the door to a room, shipping papers, or something like that. If you can find out the source activity, here’s what some of the numbers mean:
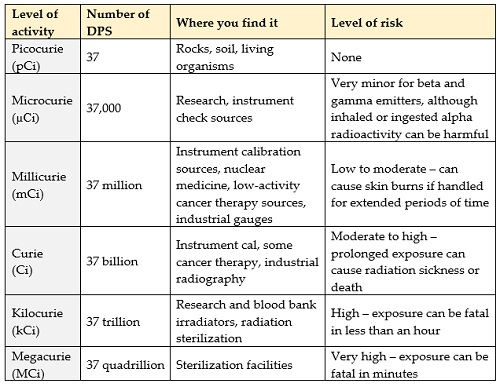
Putting it all together
So…let’s put this information to use!
- Say you’re doing a contamination survey using your trusty pancake GM and you get a reading of 2000 cpm. What should you do?
This is clearly higher than background (remember, with a GM, background is normally around 50-100 cpm), but it’s not a danger to anybody. As a radiation safety professional, I’d be inclined to clean up the contamination if I could unless it were a large-scale emergency with other more pressing problems. - During a routine radiation survey you notice radiation dose rates are around 1.5 mR/hr in a waiting room. Is this a concern?
This dose rate is clearly elevated, but not enough to pose a risk to anybody. It’s also lower than the 2 mR/hr level that would call for restricting access for members of the public. On the other hand, radiation levels are higher than they ought to be – this warrants investigation to find out what’s causing the elevated rad levels. They should be reduced if possible. - A technician tells you that an incontinent nuclear medicine patient urinated on the floor a half-hour after being injected with 10 mCi of Tc-99m. He’s measuring radiation dose rates of about 10 mR/hr with a pancake GM. What do you need to do?
Tc-99m emits a gamma with much less energy than Cs-137, so the readings we measure with a Geiger counter are going to be higher than the actual dose rates. You need to bring an ionization chamber or an energy-compensated GM to the scene to find out what the actual dose rate is before you know what actions you need to take. Oh – and clean up the radioactive urine! - You see a source lying on the ground and you’re able to find the instrument it fell out of. The label tells you that the source is 75 Ci ofIr-192. What should you do?
Seventy-five curies is a fairly high amount of activity – that much Ir-192 will produce a dose rate of about 32 R/hr a meter away, which can cause radiation sickness in 2-3 hours. This source isn’t deadly over a short period of time, but it’s got to be treated with care. You’ll need to fall back from the source until dose rates drop to 2 mR/hr and establish a radiological boundary, evacuating everyone from within that boundary (don’t forget to survey on floors above and below the source if appropriate). If you have training in recovering radiography sources then you can attempt to retrieve it. If you don’t have such training, you’ll need to contact your regulators and the manufacturer so that they can retrieve the source.