Hi, Dr. Zoomie – so…I saw something in my news feed a few days ago about scientists finding granite on the moon and now they’re saying that it’s due to extra radioactivity in the rock or something like that, and that the granite is warmer than it ought to be. It sounds very cool (or should I say “neat” since it’s actually warm?) – but I have no idea how these things go together or why scientists are even interested.
Can you explain this to me?
Thanks for including the link – it gave me a good starting point. And this is some interesting stuff! But it’s going to take a little while to explain why this is so interesting – and sort of unexpected. We’ll start off with where granite comes from…beginning with what granite actually is and how minerals form. But not to worry – we’ll get to the radioactivity part of it before too long!
Picture a lake of molten rock deep underground. It doesn’t really matter much how the rock came to melt (although it is relevant that water and plate tectonics are usually involved in the process…on Earth, anyways) – what’s important is that there’s a lake of molten rock and (more important) what happens as it starts to cool off. Just as with any cooling liquid, at the right temperature it begins to solidify. When water starts to solidify it turns to ice, but molten rock is a much more complex liquid – when magma starts to solidify it does so in stages. The first minerals to form are the ones made of small atoms; a number of them with a lot of iron, magnesium, manganese, and other metals from the central part of the Periodic Table that are called “transition elements.” These metals combine with silica to form high-temperature minerals such as olivine, and families of minerals called pyroxenes and amphiboles. The prevalence of transition metals in the crystals gives these minerals a dark color, while the relatively small size of the atoms of which they are comprised tends to exclude larger atoms from the crystal structure. I know – fascinating, right?!
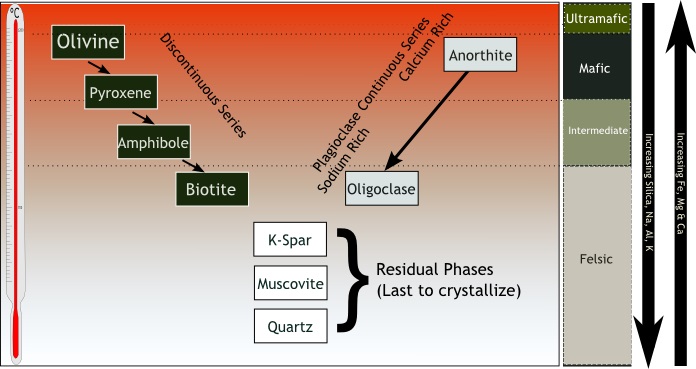
Where it gets a little more relevant to this story is that there are a lot of elements (including some metals) that are not transition metals, and these atoms tend to be on the larger side. Minerals that contain, say, calcium, sodium, and potassium tend to be red, pink, gray, and white instead of the black, olive green, and other dark colors we saw earlier. It is these light-colored minerals that are found in true granites[1].

As to why this is important…uranium, thorium, and potassium are large atoms and they are radioactive (all uranium and thorium atoms are radioactive; with potassium it’s only one atom in 10,000 that is radioactive potassium-40). Not only that, but uranium and thorium decay to stable isotopes of lead only after passing through many radioactive intermediaries (14 for U-238 and 10 for U-235 and Th-232). Or, to put it another way, the minerals that make up granite contain large atoms, including the ones that are naturally radioactive.
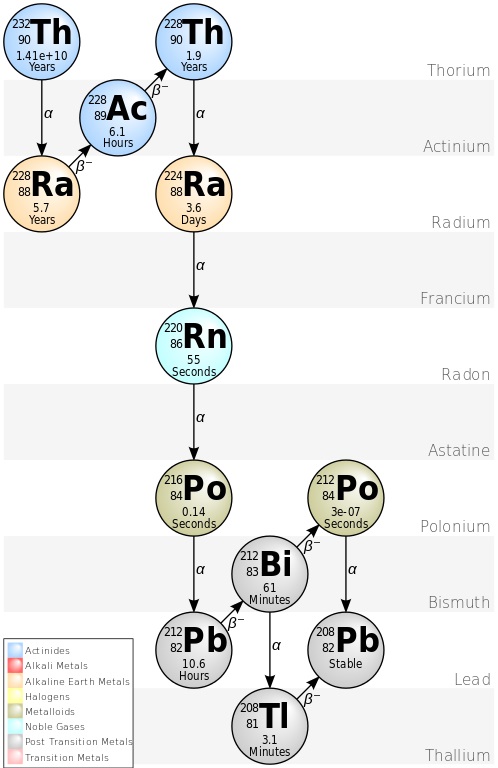
Every radioactive atom that decays emits energy and most of this energy is absorbed by the rock in which the atoms are found. Every atom of U-238 dumps more than 4 million electron volts (MeV) into the rock and its radioactive decay progeny add another 46 or so MeV. The other radioactive elements all add their own share to the energy deposited in the rock – a large piece of granite (we’re talking cubic miles of rock, not your kitchen counter) with enough U, Th, and K will produce enough heat to detect from a distance. And that brings us to the Moon!
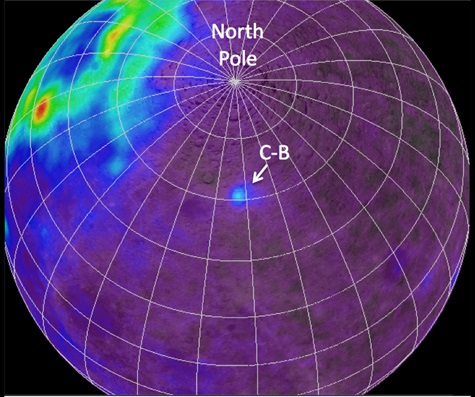
In October, 2007 the Chang’e 1 lunar orbiter launched from China’s Xichang Satellite Launch Center, followed by a companion (Chang’e 2) three years later. Among their instruments are sensors that let planetary scientists measure the amount of heat present in the rocks beneath. A recent paper in the scientific journal Nature (https://www.nature.com/articles/s41586-023-06183-5) reports that the heat emanating from a rock formation on the far side of the Moon is giving off much more thermal energy than expected. Further study suggests that this thermal anomaly can be explained if the rock formation (called Compton-Belkovich) is laced with thorium – as much as 20 times as much as typical lunar rocks. And, as it turns out, this area has been known to be thorium-rich for a quarter-century, the thorium was discovered by an orbiting gamma spectrometer in 1998. In fact, the concentrations of thorium at this location are nearly 100 times as high as typical concentrations on Earth.
Incidentally (in case you’re curious), even this much thorium is not going to give future astronauts (or thorium miners) health problems; annual radiation exposure would be lower than what radiation workers are permitted to receive, and are comparable to what many medical professionals receive from the radiation they administer to their patients while performing (for example) any of a number of cardiology procedures on a regular basis.
So a quick summary of the main scientific issue. There’s a question as to how granite formed on the Moon in the absence of water and plate tectonics – having said that, we’ve identified ice on the Moon so we know there’s water there, so it’s plausible to speculate that an episode of volcanic activity in the vicinity of an ice deposit could well have produced some granite. And there are other areas on the Moon in which large atoms have been concentrated into rock formations, so we know that this happens as well. All of which means the presence of a thorium-rich deposit of granite seems plausible, even on the Moon…which is good since there’s fairly good proof that one exists!
And one final thought…. The Moon contains uranium as well as thorium, which means that we might well find places with useful amounts of uranium as well. Not only that, but the lunar soil seems to have collected large amounts of helium-3, which has been proposed as fuel for future fusion reactors. With uranium, thorium, and He-3 it seems the Moon might be in good shape for producing the energy it will need during the long lunar nights, should we ever get around to building a city on the Moon.
Image References:
- https://www.nps.gov/yose/learn/nature/granite.htm
- The Compton–Belkovich Thorium Anomaly’s location on the Moon.
- Bowen’s Reaction Series
- https://commons.wikimedia.org/wiki/File:Decay_Chain_of_Thorium.svg
[1] “Black granite” is not what a geologist would call granite – it’s what an interior designer calls basalt or diorite.